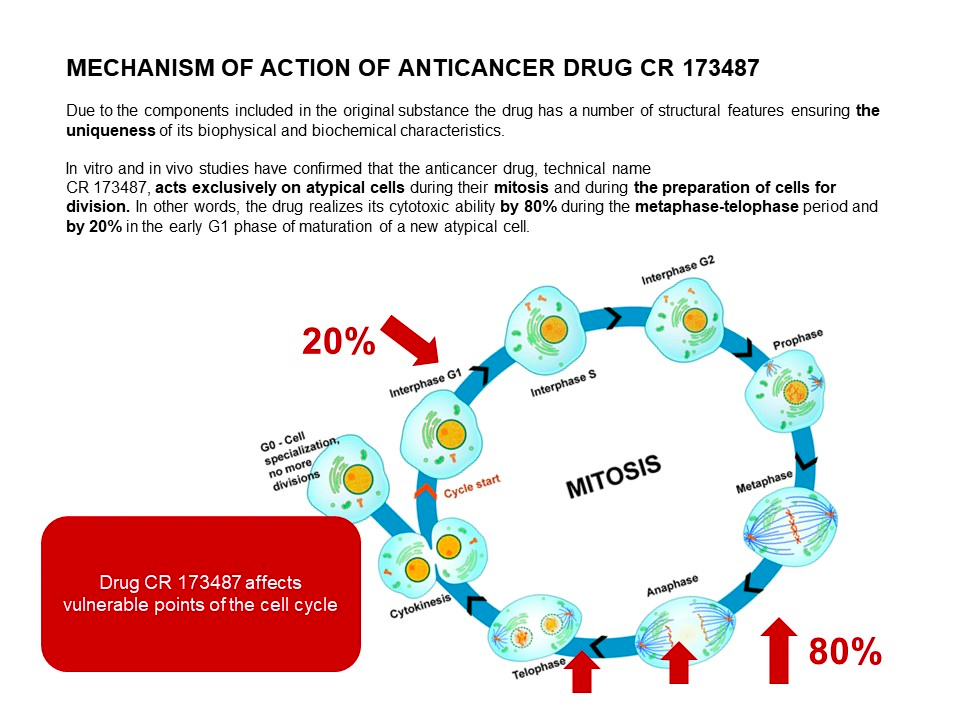
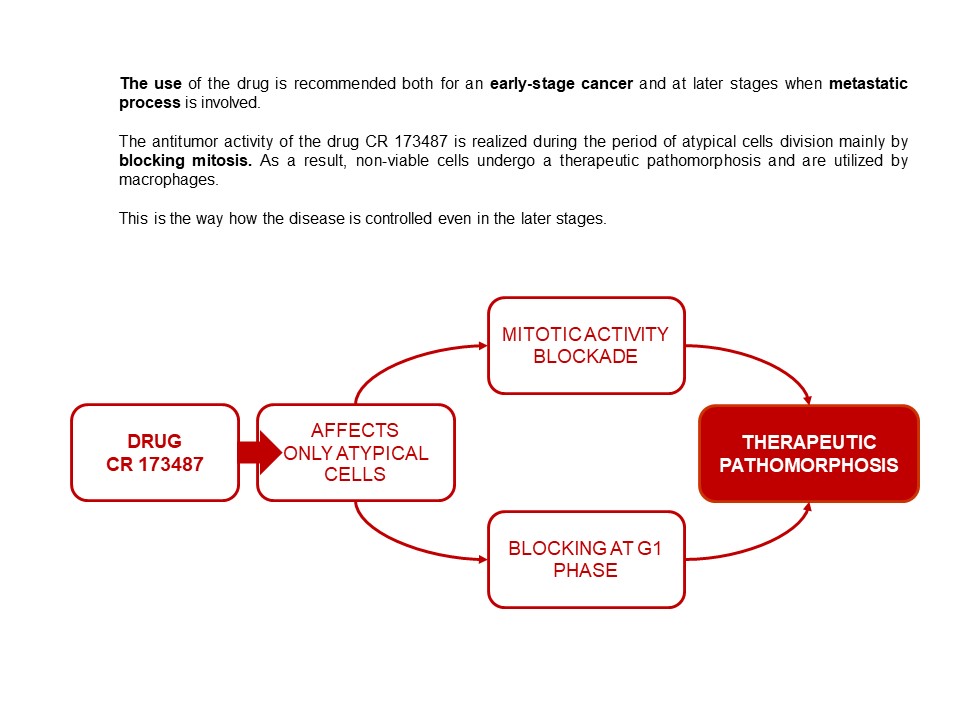
INFORMAION ABOUT ANTITUMOR DRUG CR 173487 FOR HEALTHCARE PROFESSIONALS
Study of the drug
Studies of drug CR 173487 has been carried out in the leading research institutes of Ukraine and in medical institutions on such parameters as tolerance, safety, therapeutic action, pharmacokinetic and pharmacodynamic characteristics and therapeutic dosage levels and the study of ways its administration, development of treatment protocols according to the diagnosis has been made as well.
Phase I of clinical studies, 2007- 2009
| Subjects | Diagnoses | Medical Institutions/ Head of Research | Dosage form |
Tolerance Acute toxicity of the drug | Brain Tumors: • Anaplastic Oligodendroastrocytoma; • Anaplastic Astrocytoma; • Ependimoma; • Gliosarcoma; • Glioblastoma; | Chief neuro-oncologist of NAMS of Ukraine, head of the Pediatric neuro-oncology and neurosurgery department at A.P. Romodanov institute of NAMS of Ukraine, corresponding member of NAMS of Ukraine, professor, Doctor of Medical Science, Vladimir Rozumenko | Solution for injections |
The studies of antitumor drug CR 173487 allowed for preliminary conclusions on anticancer activity of the drug as to:
- clinical inhibition of the tumour process
- process of therapeutic pathomorphosis
- the ability to improve of quality of life
Since 2018, clinical trials of the drug CR 173487 have been carried out on a number of the following aggressive tumours:
| Intracerebral tumours | Gastrointestinal tumours (GI Tract) | Endocrine tumours | Lungs’ cancer |
Due to advanced research conducted during the clinical trials, Phase I, the following parameters were recorded and approved:
- maximum allowable doses of the drug (tolerance)
- dosage of drugs necessary for the treatment of a particular cancer type
- the method of administration of the drug (form of use)
Phase II of Clinical Trials
Phase II of controlled randomized clinical trials showed high effectiveness of the drug and allowed to draw up preliminary treatment protocols depending on the diagnosis and other important indicators.
Phase III of Multicenter Studies and Clinical Trials
Phase III of clinical trials and studies has been actively conducted on today and it is characterized by positive qualitative results.
The results of the studies already carried out indicate the non-toxicity and safety of the drug. In other words, the drug has no toxic effect on the human body. Its use is safe for patients; the facts of the direct toxic effect of the drug on the human body have not been recorded yet.
The reaction to drug administration is often manifested by a slight increase in the patient’s body temperature during the first 3-5 days after the drug administration, which indicates a response of the body’s immune system and does not require special treatment.
Based on the results of the studies carried out, it is possible to draw preliminary conclusions about the antitumor activity of the drug; clinical inhibition of the tumour process has been recorded, which indicates in favour of therapeutic pathomorphosis and is resulted in improvement in the quality of life (according to WHO questionnaires).
No addiction to the drug is defined.
Antitumor Drug CR 173487 is able to put the patient into long-term remission and cure the patient.
Specificity of the Drug Use
Use of the drug can improve health of almost all cancer patients as the destruction of a significant part of tumour cells in the patient’s body occurs during the first two-three weeks after the beginning of the treatment.
The Length of Treatment with the Drug
The length of treatment with the drug including rehabilitation processes (removal and resorption of destroyed cells and restoration of proper functions of the patient’s body) depends on the nature and severity of the disease. For patients weakened by the previous use of highly toxic drugs and procedures and having several areas of cancer, the process of treatment with the drug is longer and is carried out in stages. The specific duration of treatment with the drug, the dose of the drug and the methods of its administration depend on the localization, histogenesis and cytogenesis of the malignant tumour.
During treatment with the drug, constant clinical control of the patient’s health is required. It is important to exclude other drugs not agreed to use including alcohol-containing drugs. The drug must not be used simultaneously with radiation and chemotherapy therapy as these methods significantly increase the level of toxic substances in the body, oppress the patient’s immune system, negatively affect the body’s ability to regenerate tissues and levels the effect of the drug.
The interval between taking the drug and other methods of influencing the tumour (chemotherapy and radiation therapy) should be at least 7 days.
Dietary supplement TIOSAN® − new possibilities for patients
Pharmaceutical company Crown Farm registered a new product in 2016, it is dietary supplement TIOSAN, an oral dietary supplement intended for use as a means to optimize the treatment of malignant neoplasms (Conclusion of the state sanitary and epidemiological expertise No.602-123-29-2/2525 dated 05.12.2016., ТУ У 10.8-40893378-001:2016; protocol of expertise No.3/8-А-3811-17-64752Е dated 31.07.2017).
Clinical research on the use of dietary supplements TIOSAN in patients diagnosed with glioblastoma and anaplastic glioma (2 clinical trial protocols) has been made at A.P. Romodanov Institute of Neurosurgery of the Academy of Medical Sciences of Ukraine starting from December 2017 and to date.
| Subjects | Diagnoses | Medical Institutions/ | Form of administration |
| Tolerance and efficacy | ● glioblastoma ● anaplastic glioma (anaplastic astrocytoma, anaplastic oligodendroglioma, anaplastic oligostrocytoma) | Chief neuro-oncologist of NAMS of Ukraine, head of the Pediatric neuro-oncology and neurosurgery department at A.P. Romodanov institute of NAMS of Ukraine, corresponding member of NAMS of Ukraine, professor, Doctor of Medical Science, Vladimir Rozumenko | Solution for per oral administration |
Preliminary results indicate the positive effect of this product on the course of treatment of intracerebral III and IV cancer stages (degrees of malignancy).
TIOSAN improves the quality of life of patients and it does not cause side effects and is not toxic.
The reaction to the product is often manifested by a slight increase in the patient’s body temperature in the first 3 to 5 days after the administration, which indicates a response of the immune system of the body and does not require special treatment.
Addiction to the product has not yet been recorded.
TIOSAN is safe for to use.
Data on its effectiveness has been accumulating.
TIOSAN can be used in the treatment of various cancer processes.